2018 WAEC Physics Specimen Expo & Apparatus
Below are WAEC Physics Specimen Expo 2018
Question 1
A particle is dropped from a vertical height h and falls freely for a time t. With the aid of a sketch, explain how h varies with
(a) t;
This question on projectile motion was well attempted by candidates although the performance was generally low because many candidates did not indicate the origin (0, 0). They also could not relate how h varies with t and t2.
Observation
The expected answers are as shown below:
(a) Diagram
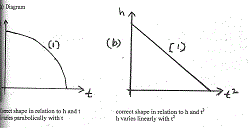
Correct shape in relation to h and t correct shape in relation to h and t2
h varies parabolically with t h varies linearly with t2
Question 2
A particle is projected horizontally at 15ms-1 from a height of 20m.
Calculate the horizontal distance covered by the particle just before hitting the ground.
[g = 10 ms-2]
Many candidates gave acceptable responses to register high performance. However, few failed to apply the appropriate formula.
Observation
The expected answer is:
Let R represent the horizontal distance covered at time, t.
h = ½ gt2
20 = ½ x 10 x t2
t = 2 s
R = ut
= 15 x 2
= 30m
Question 3
Explain why mercury does not wet glass while water does.
Observation
Good responses were given by many candidates. Few fumbled due to their failure to relate cohesion and adhesion forces with molecules of mercury and glass.
The expected answers are:
Mercury does not wet glass because (force of) cohesion/attraction of mercury molecules is greater than (force of) adhesion/attraction between glass and mercury molecules.
Water wets glass because (force of) adhesion/attraction between glass and water molecules is greater than (force of) cohesion/attraction of water molecules.
Question 4
Explain what is meant by cations.
Draw and label an electrolytic cell
Observation
(a) Many candidates gave an incomplete explanation. Many stopped at “cations are positive ions” and not all positive ions are cations.
(b) Many candidates were unable to present a diagram that shows a cathode and an anode dipped in an electrolyte or they drew wrong diagrams connecting external components to an otherwise correct set-up.
Very poor performance was registered by candidates.
The expected answer is:
(a) Cations – are positive ions that are attracted to the cathode (during electrolysis) (b) Diagram
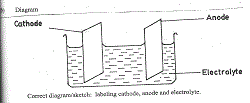
Correct diagram/sketch: labeling cathode, anode and electrolyte.
Question 5
State three methods of polarizing an unpolarized light.
Observation: This question on polarization was very popular among the candidates and their responses were quite commendable.
Question 6
State Faraday’s second law of electrolysis.
An electric charge of 9.6 x 104 C liberates 1 mole of substance containing 6.0 x 1023 atoms. Determine the value of the electronic charge.
Observation: In part (a), many candidates were able to state the law correctly. Candidates’ performance in part (b) was poor due to lack of knowledge of the appropriate formula.
The expected answers are as follows:
(a) If the same quantity of electricity is passed through different voltameters/electrolytes connected in series the masses of the substances liberated/deposited during electrolysis is (directly) proportional to their chemical equivalents.
OR The mass of an element deposited/liberated during electrolysis is (directly) proportional to the chemical equivalent of the element.
(b) Let e represent the electronic charge.
e = Faraday’s constant
Avogadro’s number
= 9.6 x 104
6. 0. x 1023
= 1.60 x 10-19C
Question 7
Explain the following terms:
tensile stress;
Young’s modulus
Observation: Explanation of tensile stress and Young’s modulus were poorly stated by majority of the candidates. While many scored for valid additional information, many others failed to relate tensile stress with a wire or rod. The expected answers are:
(a) Tensile stress is the ratio of force to cross-sectional area of a wire/rod It is expressed in Newton per metre squared (Nm-2)
(b) Young’s modulus is the ratio of tensile stress to tensile strain. It is expressed in Newton per metre squared. (Nm-2)
Question 8
a) Define diffusion.
(b) State two applications of electrical conduction through gases.
Observation: This question was poorly answered by most of the candidates that attempted it. Low performance was recorded particularly in part (a) where many candidates mistook diffusion for osmosis. The expected answers are:
(a) Diffusion is the process by which substances mix (intimately) with one another due to the random motion of their molecules.
(b) Applications of electrical conduction through gases include:.
- In advertising industry/Neon signs
- In lighting/fluorescent tubes
- Identification of gases
- Cathode ray oscilloscope/T.V. tubes
Question 9
a) List two properties of cathode rays.
(b) Explain how the intensity and energy of cathode rays may be increased
Observation: Part (a) was fairly popular with the candidates but those who wrote cathode rays travel in a straight line did not earn mark for it because they were expected to add in “field free space” to earn the point. Part (b) was not well attempted. The expected answers are:
(a) Properties of cathode rays:
Cathode rays
Are negatively charged.
Travel in straight line in field free space.
Are deflected by electric/magnetic field.
Possess(kinetic) energy.
Possess momentum.
(b)(i) The intensity of cathode rays may be increased by raising the temperature of the cathode/increasing the current through the heater.
(ii) The energy of cathode rays may be increased by raising the potential difference between
the anode and the cathode/the anode potential.
Question 10
Give three observations in support of de Broglie’s assumption that moving particles behave like waves.
Observation: Few candidates attempted this question but, majority of those who did, performed satisfactorily.
Question 11
(a) Given a retort stand and clamp, a stout pin, a simple pendulum and a pencil, describe how you would use these apparatus to determine the centre of gravity of an irregularly shaped piece of cardboard of a moderate size.
(b) Using a suitable diagram, explain how the following can be obtained from a velocity-time graph:
(i) Acceleration;
(ii) Total distance covered.
( ) A body at rest is given an initial uniform acceleration of 6.0 ms-2 for 20 s after which the acceleration is reduced to 4.0 ms-2 for the next 10 s.
The body maintains the speed attained for 30 s.
Draw the velocity-time graph of the motion using the information given above. From the graph, calculate the:
Maximum speed attained during the motion;
Total distance traveled during the first 30 s;
Average speed during the same time interval as in (ii) above.
Observation: This question was very popular among the candidates.
Performance in part (a) was poor because many candidates ended up describing the simple pendulum experiment. Performance was average in part (b). In part (c) performance was poor because majority of the candidates failed to present acceptable/correct sketches. Hence they could not validly make the deductions sought.The expected answers are as follows:
(a) PROCEDURE
Make at least 3 well-spaced pin holes round the edge of the cardboard. Clamp the pin horizontally and suspend the cardboard on it through one of the pin-holes such that the cardboard can swing freely
Hang the simple pendulum on the same pin and let its string be very close to the cardboard.
When the whole system is at rest (or in equilibrium) trace the plumbline on the cardboard. Repeat the procedure for each of the two other pin holes.
CONCLUSION
The point at which the (three) traced lines intersect is the centre of gravity of the cardboard.
PRECAUTIONS
Repeat procedure
Pin rigidly and firmly held by retort stand and clamp
Allow the simple pendulum to rest before tracing the shadow of the plumbline on the cardboard.
The string to be close to the cardboard
11(b) Diagram:
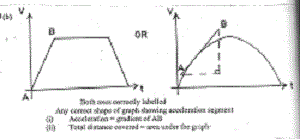
Both axes correctly labelled
Any correct shape of graph showing acceleration segment
Acceleration = gradient of AB
Total distance covered = area under the graph
11(c) Diagram
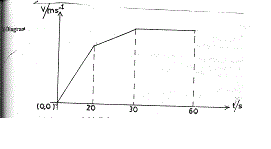
At least one axis labelled
Correct shape of graph
Let V1 = maximum velocity after 20 sec.
V2 = maximum velocity after 30 sec.
(i) Then V1 = 6
20
∴ V1 = 120 ms-1
Also V2 – V1 = 4
10
V2 – V1 = 40
V2 = 120 + 40
V2 = 160 ms-1
Maximum speed = V2 = 160ms-1
(ii) Total distance covered = Area of Δ + Area of trapezium after 1st 30 seconds
= ( ½ x 20 x 120) + ½ (120 + 160) x 10
= 1200 + 1400
= 2600m
(iii) Average speed = Total distance
Total time
= 2600 30
= 86.67 ms-1
Question 12
(a) Explain why it is not advisable to sterilize a clinical thermometer in boiling water at normal atmospheric pressure.
(b) State the effect of an increase in pressure on the
(i) boiling point; and
(ii) melting point of water.
(c) Diagram:
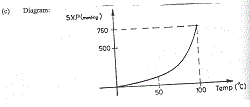
The graph shown above is that of the saturated vapour pressure (s.v.p.) of water against temperature.
Pure water is known to boil at 100oC and at an atmospheric pressure of 760 mmHg. What general conclusion can be drawn from the information given above?
d) A thread of mercury of length 20 cm is used to trap some air in a capillary tube with uniform cross-sectional area and closed at one end. With the tube vertical and the open end uppermost, the length of the trapped air column is 15cm. Calculate the length of the air column when the tube is held:
i) horizontally;
ii) vertically with the open end underneath. [Atmospheric pressure = 76 cmHg ]
Observation: Generally, candidates’ responses fell short of expectation in part (a) but performance was very high in parts (b) & (d). Majority of candidates did not answer part (c) of the question. The few that gave responses to it did more of guess work than state the required general conclusion.
The expected answers were as follows:
(a) A clinical thermometer has small temperature range. The glass will crack/burst due to excessive pressure created by expansion of mercury.
(b) Increase in pressure
(i) increases the boiling point;
(ii) decreases the melting point.
At the boiling point of pure water, the saturated vapour pressure (s.v.p)
of the water is equal to the external atmospheric pressure.
(d) Diagram
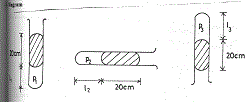
Volume V is proportional to length Ɩ stated or implied
(i) P1V1 = P2 V2 implies P1 Ɩ1 = P2Ɩ2
(76 + 20) 15 = 76 x Ɩ2
Ɩ2 = 96 x 15
76
Ɩ2 = 18.95 cm
(ii) P1 Ɩ1 = P3 Ɩ3
(76 + 20) 15 = (76 – 20) x Ɩ3
Ɩ3 = 96 x 15
56
Ɩ3 = 25.7l cm
