WAEC 2018 Chemistry practical Answers and Questions
Below Are WAEC Chemistry Practical Answers and Questions
Question 1
A is 0.100 mol dm-3 solution of an acid.
B is a solution KOH containing 2.8 g per 500 cm3.
(a) Put A into the burette and titrate it against 25.0 cm3 portions B using methyl orange as indicator. Repeat the titration to obtain consistent titres. Tabulate your readings and calculate the average volume of A used.
(b) From your resulsts and the information provided above, calculate the:
(i) number of moles of acid in the average titre;
(ii) number of moles of KOH in the volume of B pipetted;
(iii) mole ratio of acid to base in the reaction
[H = 1.00, O = 16.0, K = 39.0]
OBSERVATION: Part (a): this question was well attempted by most candidates and the performance was quite good. The table and average titre value were correctly done by majority of the responding candidates. Few candidates however, had no table of titres while majority of others altered and/or cancelled their tables to make their titre values correspond with the teacher’s titre.
In part (b), most candidates were unable to calculate neither the number of moles of the acid nor the number of mole of the base in 25.0cm3 and hence they were unable to determine correctly the mole ratio of acid to base. The expected answers are:
(b)(i) number of moles of acid = 0.100 x VA
1000
= X mole(s) [3sig. Fig to score]
1000cm3 contains 0.100 mole(s)
VA will contain 0.100 x VA
1000
= X moles [3 Sig. Fig. to score]
(ii) Number of moles of KOH in B
500cm3 of B contains 2.8g of KOH
1000cm3 of B will contain 2.8 x 1000 = 5.6 KOH
500
Molar mass of KOH = 39 + 16 + 1 or 56 gmol-1
Conc of B = 5.6 = 0.100 mol dm-3
56
OR
Molar mass of KOH = 39 + 16 + 1 or 56 gmol-1
Number of moles of KOH = 2.8 = 0.05 mole(s)
56
500cm3 contains 0.05 mole(s)
1000cm3 will contain 0.05 x 1000
500
= 0.100 moldm-3
Number of moles of KOH in B = 0.100 x 20/25 = 0.0025 mole(s)
1000
= Y mole(s)
(iii) Mole ratio of acid to base = X: Y to nearest whole number ratio
Question 2
C is a mixture of two salts. Carry out the following exercises on C. Record your observations and identify any gas(es) evolved. State the conclusion drawn from the result of each test.
Put all of C into a boiling tube and add about 5cm3 of distilled water. Stir thoroughly and filter. Keep both the residue and the filtrate.
To about 2 cm3 of the filtrate, add few drops of Pb(NO3)2(aq)‑Boil the mixture and then allow to cool.
(i) Put the residue in a test tube and add dilute HNO3. Shake the mixture and divide the solution into two portions.
(ii) To the first portion from (c)(i), add NaOH(aq) in drops and then in excess.
(iii) To the second portion from (c)(i), add aqueous ammonia in drops and thein in excess.
OBSERVATION:Adding distilled water to the specimen in a boiling tube and making accurate observations were successfully done by most candidates. Few candidates however were not able to give logical deductions from their observation while some could neither carry out the tests as prescribed nor were able to record their observations. It was observed that many candidates still confused residue with precipitate and or solid. Other mistakes include: white ppt after filtration instead of white residue; clear filtrate instead of colorless filtrates; ppt disappears instead of ppt dissolves; white chalky ppt instead of white gelatinous ppt and carrying out the test on the dry sample instead of solution as demanded by the question.
TEST
OBSERVATION
INFERENCE
a) C + water, mixture stirred
and filtere
Partly dissoves/soluble Colourless filtrate
White residue (do not accept ppt/solid)
C contains soluble and insoluble salts
b) Filtrate + Pb (NO3)2(aq)
Boiled
then cooled
White precipitate formed
Precipitate dissovles
Precipitate reappeares
Cl-, SO42- or CO32-any for (2 marks) (SO32- do not score but do not penalize)
Cl- present
Cl- confirmed
c) (i) Residue + dil HNO3
Effervescence/bubles/gas evolved colourless odourless gas. Gas turns lime water milky.
CO2 evolved from CO32-
(ii) Solution from (c)(i) +
NaOH(aq) in drops then in
excess
White gelatinous ppt formed
Precipitate dissovles
Zn2+ or A13+
Zn2+ or A13+
(iii) Solution from (c)(i) +
HN3(aq)
in drops
then in excess
White gelatinous
Precipatate form
Precipitate dissovles
Zn2+ or A13+
Zn2+ confirmed
Question 3
A colourless gas P was given off when dilute tetraoxosulphate (VI) acid was added to zinc salt Q. On bubbling the gas through lime water, a white precipitate R was formed. Identify P, Q and R. Name a suitable apparatus that could be used to perform each of the following activities in the laboratory:
i. storage of dilute silver trioxonitrate (V);
ii. heating copper metal;
iii. separation of a mixture of water and kerosine.
Give one reason for each of your answers in (b).
Most candidates were unable to make precise statements. Most candidates correctly identifiedPas CO2 and R as CaCO3 but could not identify Q as ZnCO3. Most candidates could neither name nor give correct reason for a suitalbe apparatus for:
storing of dilute silver trioxonitrate (V);
heating copper metal.
OBSERVATION: Many candidates however, correctly named the separating funnel and gave correct reason for using it. The expected answers are:
P – CO2 or Carbon(IV) oxide
Q - ZnCO3 or Zinc trioxocarbonate (IV)
R – CaCO3 or calcium trioxocarbonate (IV)
(i) Dark brown/amber coloured bottle
(ii) Bunsen burner
(iii) Separating funnel
Reason
Amber coloured bottle reduces the intensity of/prevents light entering the bottle to decompose Ag NO3.
Bunsen burner produces high intensity of heat/temperature
Because water and kerosene are not miscible/due to immiscibility of water and kerosene.
Question 4
D is a solution of 0.100 mol dm-3 HNO3.
E is a solution containing 13.6 g of Na2CO3 yH2O perdm-3
Put D into the burette and titrate it against 20.0 cm3 or 25.0 cm3 portions of E using methyl organge as indicator. Repeat the titration to obtain consistent titres. Tabulate your readings and calculate the average volume of D used. The equation for the reaction involved in the titration is:
2HNO3(aq) + Na2CO3.yH2O(aq)→Na2CO3(aq)+ CO2(g)+ (y+1) H2O(1)
(i)From your results and the information provided above, calculate the:
concentration of E in mol dm-3;
concentration of E in g dm-3;
value of y in Na2CO3.yH2O.
[H = 1.00, C = 12.0, O = 16.0; Na = 23.0]
OBSERVATION: In part (a) candidates correctly tabulated their burette readings and calculated the average titre value. Some candidates however manipulated their readings to agree with those of their teachers. In part (b) (i) and (ii), most candidates that lost marks in this section did so for either using wrong units or not expressing their answers to three significant figures. In section (iii), many candidates correctly calculated the molar mass of Na2CO3 and obtained the mass of anhydrous salt. In the calculation of the value of y however, many candidates failed to express their final answer to the nearest whole number while others muddled up the substitution . Performance was on the average. The expected answers are:
(b)(i) CDVD = 2 mole ratio
pic CEVE 1
CE = CDVD making CE subj
2VE
= X moldm-3
(b(i)Amount of D used = 0.100 x VD = d mol
1000
From the balanced equation of the reaction
2 moles of D = 1 mole of E
D mol of D = d mole of E
2
i.e 20/25 cm3 of E contains d/2 mol
1000 cm3 of contained d x 1000 mol
2 x 20/25
= e mol
Conc. of E = e moldm-3
Molar mass of Na2CO3 = 106 gmol-
Mass of anhydrous Na2CO3 = 106 x X moldm-3
&nbs
Question 5
F is a mixture of two compounds. Carry out the following exercises on F. Record your observations and identify any gas(es) evolved. State the conclusion drawn from the results of each test.
Put all of F into a beaker or a boiling tube and add about 10 cm3 of distilled water. Stir the mixture thoroughly and filter. Keep both the filtrate and the residue.
(i) Divide the filtrate into two portions.
I.To the first portion, add dilute HNO3 followed byAgNO3(aq).
II. Add excess NH3(aq) to the resulting mixture.
(ii) To the second portion, add NH3(aq) in drops and then in excess.
Add about 5 cm3 of distilled water to the residue in a test tube. Boil and allow to cool. Add few drops of iodine solution.
OBSERVATION: Majority of the responding candidates carried out the required tests, made their observations and drew inferences in the prescribed format. However, some candidates lost marks for not reporting that no visible reaction was observed on adding HNO3 to the filtrate and for including pb2+ as ions present when the filtrate was treated with NH3(aq)
Observation of jelly-like paste was also not recorded by many candidates when water was added to residyue and then heated.
On addition of iodine to the cooled mixture, most candidates recorded black ppt instead of blue-black colouration. General performance was fair. The expected answers are:
TEST
OBSERVATION
INFERENCE
a) F + water, stirred mixture and filtered
Partly dissoves/soluble Colourless filtrate
b) (i) I
White residue
F contains soluble and insoluble salts
b) (i) I
Filtrate + dil HNO3
II
Filtrate + dil HNO3
+ Ag NO3(aq)
Mixture in (b)(i) +
excess NH3 solution
No visible reaction
White precipitate formed
Precipitate dissovles
Cl- present
Cl- confirmed
b)(ii)
Filtrate + HN3(aq) in drops
then in excess
White gelatinous precipitate
Precipitate dissovles
Zn+2 or A13+
may be present
Zn2+ confirmed
c)
Residue + water + heat
allow to cool + iodine solution
Jelly-like paste is formed
Dark/deep blue/blue black colouration is obtained
Starch is confirmed
Question 6
The following diagram is a set-up for the separation of a mixture of palm oil and water:
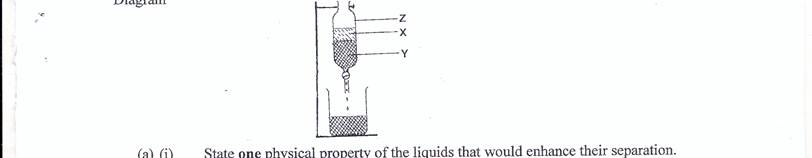
pict
Diagram
(i) State one physical property of the liquids that would enhance their separation.
(ii) Identify X;Y.
(iii) Name the apparatus labelled Z.
Name two basic apparatus used in each of the following separation methods:
fractional distillation;
paper chromatography;
evaporation.
(i) What is the color of phenolphthalein in aqueous ammonium chloride?
(ii) State what would be observed on:
bubbling chlorine through iron (II) chloride solution;
passing ethene through bromine water
OBSERVATION: Part (a) candidates performed well in this question. Part (b) many candidates could not answer this part correctly probably due to lack of laboraory equipment. Part (c) was also poorly done by most candidates as they could not state correctly the colour changes as required by the question. The expected answers are:
(a)(i) Immisciblility (accept not miscible)
(ii) I. X - palm oil
II. Y - water
(iii) Z - separating funnel
(b)(i) fractionating column, liebig condenser, thermometer, source of heat, retort standwith clamp, distillation flask.
(ii) glass cover/split cork, tank/boiling tube/beaker, filter paper/plate
(iii) evaporating basin/dish/beaker, tripod stand with wire gauze, source of heat/bunsen burner.
(c)(i) colourless
(ii) I . the green iron (II) chloride changes to brown
II. bromine water being brown changes to colourless/brown colour of bromine water decolorised.
